Over 55 Years of Photobiomodulation Therapy Research Reveals Remarkable, Consistent Results
The efficacy of Photobiomodulation Therapy has been well documented since its discovery in 1955. Below are just a few of the articles and papers published on Photobiomodulation Therapy as a viable modality for various conditions. Key findings in a study published by NASA in 2001 stated that:
- “LED produced in vitro increases of cell growth of 155-171% of normal human epithelial cells.”
- “LED produced improvement of greater than 40% in musculoskeletal training injuries in Navy SEAL team members, and decreased wound healing time in crew members aboard a U.S. Naval submarine.”
- “LED produced a 47% reduction in pain of children suffering from oral mucositis.”
Overall NASA Concluded:
“We believe that the use of NASA LED for light therapy alone, and in conjunction with hyperbaric oxygen, will greatly enhance the natural wound healing process, and more quickly return the patient to a preinjury/illness level of activity.” Read the Complete NASA Study. The video below is a clinical trial with cancer patients based on NASA research.
Photobiomodulation Therapy (Light Therapy) is not new. The barrier to widespread use has been the lack of a device that is both powerful enough for fast results and affordable – until now. Bales Photonics Firefly brings this non-invasive therapy option to doctors and integrative practitioners at a fraction of the cost.
Advances in Pain Management with Photobiomodulation Light Therapy
by Len Saputo, MD, with Jerry Stine, NC
The 2019 Townsend Letter documents Dr. Len Saputo’s work with Firefly inventor, Maurice Bales stating, “It is increasingly clear that Photobiomodulation (PBM) is highly effective for a wide range of painful conditions that previously had few, if any, effective medical options. PBM is also safe, affordable, and available.”
“I am confident that any healthcare practitioner who has had the experiences I’ve had using light therapy would include it as a major part of their approach to managing patient pain.”
Cognitive Decline Trial Shows Promising Results
In 2019 Dr. Suzan Syed and I conducted a small study on patients diagnosed with cognitive decline in 2019-2020. With dementia affecting 5.7 million Americans and over 50 million people globally, we wanted to know first-hand if our Photobiomodulation (PBM) light therapy could help. This was a small study with patients who had a definitive diagnosis of Dementia from their neurologist.

Patients were evaluated with the F-Scan to determine frequencies they were sensitive to. The frequencies found most sensitive were then programmed into a function generator. The function generator was set to input frequency information (sinusoidal waveform, 5V peak-peak) into the Bales Photonics Firefly Clinic Pro using 37,700 mW intensity. Subjects received 10 sessions for 10 minutes over the course of 4 weeks with a minimum of 2 sessions per week using their unique frequencies.
Help for Post-Covid Issues – Read our Case Study
A single but promising case study revealed that in just two 5-minute anterior lung applications with Firefly Light Therapy the patient, a 62 year old male struggling to recover from post-COVID lung symptoms, had remarkable results.
Before COVID-19 became widespread Dr. Bales was infected with the virus and decided to try the Firefly for his loss of taste and smell. Both returned after a series of sessions. Imagine life without taste and smell?! This is a reality for many – and we can help them.

After applying Firefly Light Therapy on my father (who has Alzheimer’s and Parkinson’s disease) for about 3-4 weeks, he is more alert and responsive and his neck flexibility has improved so he can turn his head from left to right. Moreover, it has helped him swallow. I am so grateful for this therapy and believe there will be more positive outcomes as we continue Firefly sessions. Thank you! Colleen P.
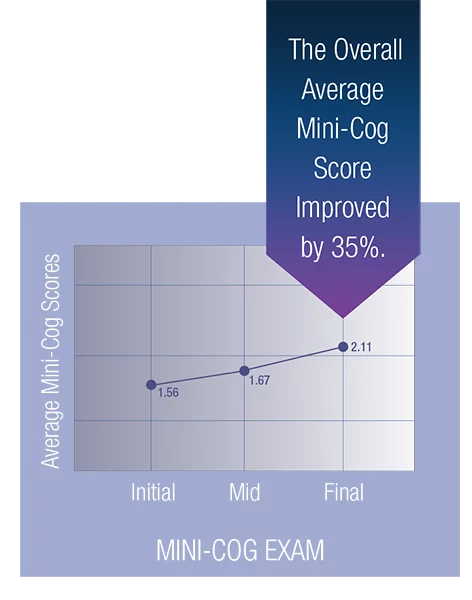
The average Mini-Cog score improved by 7% from the pre-trial to the post-trial exams. Scores improved by another 26% from the post-trial exam to the post-washout exam. The overall average Mini-Cog score improved by 35%.
Read the Research for Specific Conditions:
Shining Light On the Head: Photobiomodulation for Brain Disorders
AUTHORS: Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA.
Department of Dermatology, Harvard Medical School, Boston, MA.
Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA 02139, USA
Michael R. Hamblin: ⁎Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA 02114, USA.Wellman Center for PhotomedicineMassachusetts General HospitalBostonMA
 
Read and download the entire article here.
CONCLUSIONS AND IMPLICATIONS OF FUTURE THERAPY
The brain suffers from many different disorders that can be classified into three broad groupings: traumatic events (stroke, traumatic brain injury, and global ischemia), degenerative diseases (dementia, Alzheimer’s, and Parkinson’s), and psychiatric disorders (depression, anxiety, post-traumatic stress disorder). There is some evidence that all these seemingly diverse conditions can be beneficially affected by applying light to the head. There is even the possibility that PBM could be used for cognitive enhancement in normal healthy people.
Many investigators believe that PBM for brain disorders will become one of the most important medical applications of light therapy in the coming years and decades. Despite the efforts of “Big Pharma”, prescription drugs for psychiatric disorders are not generally regarded very highly (either by the medical profession or by the public), and many of these drugs perform little better than placebos in different trials, and moreover can also have major side-effects. Even after many years of research, no drug has yet been developed to benefit these neurodegenerative disorders. A similar state of play exists with drugs for stroke (with the exception of clot-busting enzymes) and TBI. New indications for PBM such as global ischemia (brain damage after a heart attack), post-operative cognitive dysfunction, and neurodevelopmental disorders such as autism spectrum disorder may well emerge. Table 2 shows the wide range of brain disorders and diseases that may eventually be addressed with some kind of PBM, whether that be an office/clinic-based procedure or a home-use based device.
AUTHORS: Raj Gupta, MD, PhD–Director, Ultra-high Resolution Volume CT Lab, Director, Advanced X-ray Imaging Sciences (AXIS) Center, Associate Professor of Radiology, Harvard Medical School. Benjamin Vakoc, PhD, Wellman Center for Photomedicine at Massachusetts General Hospital
CONCLUSIONS AND IMPLICATIONS OF FUTURE THERAPY
Researchers found that they could measure the effects of transcranial LLLT on the brain. The MRI studies showed statistically significant differences in the integrity of myelin surrounding the neurons of treated patients versus the control group. The study also showed the light does impact the cells. In conclusion, the findings indicate that light therapy is safe and has measurable effects on the brain, and could become the first widely accepted treatment for moderate traumatic brain injury.
AUTHORS: Raj Gupta, MD, PhD–Director, Ultra-high Resolution Volume CT Lab, Director, Advanced X-ray Imaging Sciences (AXIS) Center, Associate Professor of Radiology, Harvard Medical School. Benjamin Vakoc, PhD, Wellman Center for Photomedicine at Massachusetts General Hospital
CONCLUSIONS AND IMPLICATIONS OF FUTURE THERAPY
Researchers found that they could measure the effects of transcranial LLLT on the brain. The MRI studies showed statistically significant differences in the integrity of myelin surrounding the neurons of treated patients versus the control group. The study also showed the light does impact the cells. In conclusion, the findings indicate that light therapy is safe and has measurable effects on the brain, and could become the first widely accepted treatment for moderate traumatic brain injury.
AUTHORS: John Mitrofanis, Luke A. Henderson Department of Anatomy, School of Medical Sciences, University of Sydney, Sydney, Australia
CONCLUSIONS AND IMPLICATIONS OF FUTURE THERAPY: In conclusion, light has a considerable influence on brain activity. It does so by stimulating mitochondrial function within individual neurons either by an activation of cytochrome c oxidase, a change in the composition of interfacial water, and/or a stimulation of any accumulated chlorophyll metabolites. When light hits the neurons “flush”, it activates various intrinsic cellular mechanisms. Light is absorbed by a chromophore (accumulated chlorophyll metabolites, interfacial nanowater and/or cytochrome c oxidase) that then prompts mitochondrial activity and hence ATP levels. Such an interaction, between light and mitochondria, helps to preserve the homeostasis of neurons, maintaining their proper function when in a healthy state or aiding their survival when in distress or after damage (Hamblin, 2016; Mitrofanis, 2019). As to why light, from a transcranial approach, influences the neurons of the default mode network, we suggest that there are evolutionary links, relating to a survival strategy for the organism against any potentially threatening or dangerous situation.
Read and download the entire article here.
AUTHOR: Ali Le Vere
This article references over 29 different studies.
CONCLUSION: Contrary to conventional wisdom, brain regeneration is possible. One promising therapy that promotes neurogenesis and is effective in pre-clinical studies of Alzheimer’s and Parkinson’s is near-infrared light therapy, and it may improve other mental illnesses and neurodegenerative disorders including dementia, stroke, ALS, and traumatic brain injury as well.
Given its large margin of safety and lack of adverse effects, near-infrared light therapy should be offered as an option for patients suffering from a myriad of chronic conditions, but is especially promising for neurodegenerative diseases including Alzheimer’s and Parkinson’s and may even have future use in multiple sclerosis. Near-infrared therapy is superior to the mainstay drug treatments for these diseases since pre-clinical studies have demonstrated proof-of-concept that near-infrared either arrests or slows the underlying pathology of these disease processes and leads to the birth of new neurons, rather than merely mitigating symptoms (10).
Read and download the entire article here.
Photobiomodulation for Alzheimer’s Disease: Has the Light Dawned?
AUTHORS: Michael R. Hamblin (with 158 documented references)
Read and download the entire article here.
CONCLUSIONS AND IMPLICATIONS OF FUTURE THERAPY
The fact that PBMT may produce a large range of beneficial changes in the brain, and is without any major side effects, suggests it should be more widely tested for AD and dementia in large controlled trials. Exposing the head to light at power levels less than that received in direct sunlight (but without harmful ultraviolet wavelengths) is intrinsically safe. Any side-effects reported have been rare, mild, and transient, consisting of a slight headache, difficulty sleeping, and mild itching on the scalp. It is likely that PBM for AD will need to be continued indefinitely, as regressions have been observed when PBM treatments have ceased. Home use PBM devices can be applied by caregivers, who consistently report improvements in their own quality of life.
Why and how does light therapy offer neuroprotection in Parkinson’s disease?
Accepted 2017 Mar 27.
Copyright : © Neural Regeneration Research
Author: John Mitrofanis, Ph.D.*
Department of Anatomy F13, University of Sydney, Sydney, Australia
Conclusions: Light therapy stimulates a bespoke intrinsic mechanism that gives the neuron the best chance of survival (Hamblin, 2016) – this is exemplified further by findings indicating that patterns of neuronal survival are similar whether the light is applied either at the same time or well after the injury (Johnstone et al., 2016). That light not only helps to defend and protect healthy neurons against degeneration but also to repair and rescue distressed neurons after injury. The repair and rescue of distressed neurons are particularly relevant to the clinical reality of Parkinson’s disease, where patients first suffer degeneration and then receive therapeutic intervention. As it stands, light therapy in the experimental setting has been shown to both protect and rescue neurons from degeneration after Parkinsonian injury, something that current therapies in patients do not do; that in itself, should be an incentive for trial in the clinical setting.
A Randomized Clinical Trial of the Effectiveness of Photon Stimulation on Pain, Sensation, and Quality of Life in Patients With Diabetic Peripheral Neuropathy
Arthur Swislocki, MD, Marla Orth, MS, MPH, Maurice Bales, AA,
Jennifer Weisshaupt, BA, Claudia West, RN, MS, Janet Edrington, RN, PhD,
Bruce Cooper, PhD, Len Saputo, MD, Melissa Islas, MD,
and Christine Miaskowski, RN, PhD, FAAN
Department of Medicine, Veterans Affairs Northern California Health Care System (A.S., M.O., M.B.,
J.W., M.I.), Martinez; Departments of Physiological Nursing (C.W., J.E., C.M.) and Community
Health Systems (B.C.) University of California, San Francisco; and Health Medicine Institute (L.S.), Lafayette, California, USA
ABSTRACT: Context. Peripheral neuropathy is one of the most common complications of diabetes.
Objectives. The purpose of this study was to evaluate the effects of photon stimulation on pain intensity, pain relief, pain qualities, and sensation and quality of life (QOL) in patients with painful diabetic peripheral neuropathy.
Methods. In this randomized, placebo-controlled trial, patients were assigned to receive either four photon stimulations (n . 63) or four placebo (n . 58) treatments. Pain intensity, pain relief, and pain qualities were assessed using self report questionnaires. Sensation was evaluated using monofilament testing. QOL was measured using the Medical Outcomes Study Short Form-36 (SF-36). Multilevel regression model analyses were used to evaluate between-group differences in study outcomes.
Results. No differences, over time, in any pain intensity scores (i.e., pain intensity immediately posttreatment, average pain, worst pain) or pain relief scores were found between the placebo and treatment groups. However, significant decreases, over time, were found in some pain quality scores, and significant improvements in sensation were found in patients who received the photon stimulation compared with placebo. In addition, patients in the treatment group reported significant improvements in SF-36 social functioning and mental health scores. Findings from a responder analysis demonstrated that no differences were found in the percentages of patients in the placebo and treatment groups who received 30% or more or 50% or more reduction in pain scores immediately posttreatment. However, significant differences were found in the distribution of the changes in pain relief scores, with most of the patients in the photon stimulation group reporting a slight (28.6%) to moderate (34.9%) improvement in pain relief from the beginning to the end of the study compared with no change in pain relief (43.1%) in the placebo group.
Conclusions. Four treatments with photon stimulation resulted in significant improvements in some pain qualities, sensation, and QOL outcomes in a sample of patients with a significant amount of pain and disability from their diabetes. A longer duration study is needed to further refine the photon stimulation treatment protocol in these chronically ill patients and to evaluate the sustainability of its effects. J Pain Symptom Manage 2009;- :-e- . 2009 U.S.
Cancer Pain Relief Committee. Published by Elsevier Inc. All rights reserved.
Thermal Imaging Processor (TIP) & Photon Stimulation: A New Form of Therapy for Chronic Diabetic Medical Painful Neuropathy of the Feet
by Jacob Green, M.D., Ph.D., Earl Horowitz, D.P.M., Deborah Fralicker, R.N., D.C., William Clewell, Ph.D., George Ossi, B.S., Aerospace Minnie Briley, C.M.E.T. and Tim Luce
B.S. Pain Digest, September/October 1999, Volume 9, Number 5
ABSTRACT: Diabetic neuropathy is a common, significant, and painful condition that does not readily lend itself to simplified Photonic therapy. Patients with painful diabetic neuropathy were treated with a new entity, i.e., a photon stimulator, and this device is described. Patients and control subjects were all assessed by physiological means (high-resolution digital infrared imaging)before and after all therapy. Patients were all given the opportunity to express their own opinions as to the efficacy of treatment outcomes via use of the standard visual analogue scale (VAS). The results are noted.
Infrared Photon Stimulation: A New Form of Chronic Pain Therapy
by Jacob Green, M.D., Deborah Fralicker, R.N., D.C., William Clewell, Ph.D., Earl Horowitz, D.P.M., Tim Luce, B.S., Victor Yannacone, L.L.B., and Constance Haber, D.C.
ABSTRACT: Three diverse problems were studied, the first of which was “chronic painful diabetic neuropathy.” This was typified by cold, painful feet. Photon therapy over the acupuncture sites and over the afflicted area resulted in increased temperature and amelioration of pain in many patients. In addition, it was noted that those who became temperature coherent (we noted a wide dispersion of recorded temperatures in symptomatic patients) were associated with better assessment of the technique by the patient.The second group of “chronic myofascial pain” syndrome patients typically demonstrated an increased are of temperature in the skin, were also treated by utilization of typical acupuncture points. For the most part, clinical improvement in pain ratings were noted associated with decreased skin temperature in affected areas becoming side-to-side coherent over time. A third patient with complex regional pain syndrome type II was also treated with this technology with clinical improvement in his previously dramatically reduced skin temperature without admission of any basic symptom change. It is felt that the infrared energy creates a change in the potentiostatic electrochemical process which invokes a non-local coupling reaction in the body’s electrical system. This would also indicate a new anatomical designation of acupuncture treatment points in correspondence with the older nomenclature which was often misleading.This is the first overall reporting of a treatment utilizing the body’s own “electrical buttons” as opposed to invoking electrical change of an internal or external invasive or semi-invasive procedure.
Photon stimulation therapy for chronic regional pain syndrome: a new technique.
Green, J, Fralicker,D, Clewell, W, et al. (1999)
Complex regional pain syndrome type I, previously known as reflex asymmetry dystrophy is notoriously difficult to treat. We report on the significant temperature reduction and side-to-side symmetry noted in one patient treated with infrared photon therapy. We review recently published experience with the photon stimulator in chronic diabetic painful neuropathy and chronic pain syndrome. Significant temperature asymmetries which were the hallmark of these other disorders were likewise similarly affected. Considerations for acupuncture type of electrochemical process change in non-local coupling functions are thought to be responsible. Neuromodulation and neuroaugmentation by this technology seem to be helpful in the amelioration of this chronic painful condition.
Chronic Myofascial Pain Treated with a New Device: The Photon Stimulator – Physiological and Clinical Assessment
by Deborah Fralicker, D.C., Jacob Green, M.D., Ph.D., William Clewell, Ph.D., George Ossi, B.S., and Minnie Briley, C.M.E.T.
JMPT, Submitted April 1999
ABSTRACT: Classical spinal and peripheral acupuncture treatment points were stimulated by an FDA approved infrared photon device in the treatment of chronic myofascial pain. Favorable assessments by the patients of this new mode of photon therapy were reported for both groups. A significant reduction in the patient’s level of pain using the standard visual analog scale for pain measurement were found. A reduction of the classic hotter (spot) skin surface temperatures in the area of the myofascial complaints that the surrounding body in both groups of patients. This infrared photon therapy device appears quite acceptable for the outpatient treatments in chiropractic physicians offices, especially those with an interest in myofascial pain and knowledge of acupuncture technique.
Healing of Bone Affections and Gangrene with Low-Intensity Laser Irradiation in Diabetic Patients Suffering from Foot Infections.
Schindl, M, Schindl, A, Polzleitner, D and Schindl, L (1998)
OBJECTIVE: Evalution of low-intensity laser irradiation on the healing of bone affections and gangrene in patients suffering from diabetic microangiopathy.
DESIGN: Case-report study.
PATIENTS: Two consecutive diabetic male patients with gangrene, osteomyelitis, and bone fractures.
INTERVENTION: Helium-neon laser irradiation (36 J/cm2 ) 50 min/day.
MAIN OUTCOME PARAMETER: Healing of gangrene and corticalis lesion as well as remineralisation of bone affections.
RESULTS: Within a mean period of 14 weeks not only a complete healing of the diabetic gangrenes but also a radiographically determined reestablishment of corticalis and remineralisation of preexisting bone affections could be achieved.
CONCLUSION: We therefore conclude that low-intensity laser irradiation should be further tested as an additional beneficial therapeutic modality for the healing of gangrene and bone affections in diabetic patients.
Low-intensity laser irradiation improves skin circulation in patients with diabetic microangiopathy.
Schindl, A, Schindl, M, Schon, H, et al. (1998)
OBJECTIVE: Diabetic foot problems due to angiopathy and neuropathy account for 50% of all nontraumatic amputations and constitute a significant economic burden to society. Low-intensity laser irradiation has been shown to induce wound healing in conditions of reduced microcirculation. We investigated the influence of low-intensity laser irradiation by means of infrared thermography on skin blood circulation in diabetic patients with diabetic microangiopathy.
RESEARCH DESIGN AND METHODS: Thirty consecutive patients with diabetic ulcers or gangrenes and elevated levels of glycosylated hemoglobin were randomized by blocks of two to receive either a single low-intensity laser irradiation with an energy density of 30 J/cm2 or a sham irradiation over both forefoot regions in a double-blind placebo-controlled clinical study. Skin blood circulation as indicated by temperature recordings over the forefoot region was detected by infrared thermography.
RESULTS: After a single transcutaneous low-intensity laser irradiation, a statistically significant rise in skin temperature was noted (P < 0.001 by ANOVA for repeated measurements), whereas in the sham-irradiated control group, a slight but significant drop in temperature (P < 0.001) was found. Subsequently performed contrasts for comparison of measurements before and after irradiation revealed significant temperature increases at 20 min of irradiation time (P < 0.001), at the end of the irradiation (P < 0.001), and 15 min after stopping the irradiation (P < 0.001). In the sham-irradiated feet, the drop in local skin temperature was not significant at 20 min (P = 0.1), but reached significance at the end of the sham-irradiation procedure (P < 0.001) and 15 min after the end of sham irradiation (P < 0.001).
CONCLUSIONS: The data from this first randomized double-blind placebo-controlled clinical trial demonstrate an increase in skin microcirculation due to athermic laser irradiation in patients with diabetic microangiopathy.
Diabetic neuropathic foot ulcer: successful treatment by low-intensity laser therapy.
Schindl, A, Schindl, M, Pernerstorfer-Schon, H, et al. (1999)
OBJECTIVE: To evaluate the efficacy of low-intensity laser irradiation for the induction of wound healing of a diabetic neuropathic foot ulcer.
CASE: We report a case of a man with insulin-dependent diabetes mellitus, sensory neuropathy, macroangiopathy and microangiopathy who had been suffering from an ulcer of his first left toe accompanied by osteomyelitis for 6 weeks.
RESULTS: After a total of 16 sessions of low-intensity laser therapy using a 670-nm diode laser administered within a 4-week period the ulcer healed completely. During a follow-up period of 9 months, there was no recurrence of the ulcer even though the patient’s metabolic condition remained unstable.
CONCLUSIONS: Although laser therapy was not applied as a monotherapy, the present observation suggests that it might constitute a useful side-effect-free alternative treatment modality for the induction of wound healing of neuropathic ulcers in diabetic patients. Therefore large properly controlled randomized studies seem justified.
Induction of complete wound healing in recalcitrant ulcers by low-intensity laser irradiation depends on ulcer cause and size.
Schindl, M, Kerschan, K, Schindl, A, et al. (1999)
Chronic skin ulcers still represent a therapeutic challenge in dermatology. Among the various non-invasive treatment modalities used for the improvement of impaired wound healing, low-intensity laser irradiations are gaining an increasing body of interest. We used low-intensity laser irradiations delivered by a 30 mW helium-neon laser at an energy density of 30 J/cm2 three times weekly for the induction of wound healing in ulcers of diverse causes. Twenty patients with the same number of ulcers, which had previously been treated by conventional wound care for a median period of 34 weeks (range: 3-120 weeks) without any significant evidence of healing, were included in the study. Concerning the underlying disorders, patients were divided into four groups: diabetes, arterial insufficiency, radio damage and autoimmune vasculitis. In all ulcers, complete epithelization could be induced by laser therapy. No amputation or any other surgical intervention was necessary and no adverse effects of any kind were noted during low-intensity laser treatment. Regarding the different diagnoses, a statistically significant difference was noted (P = 0.008): ulcers due to radio damage healed significantly faster than those caused by diabetes (6 weeks [range: 3-10 weeks] vs. 16 weeks [range: 9-45 weeks], P = 0.005). Wound healing in autoimmune vasculitis (24 weeks [range: 20-35 weeks]) required longer than in radiodermitis, although the difference was not significant. In addition to the diagnosis, wound size was found to be an important factor influencing the duration of wound closure (P = 0.028), whereas duration of previous conventional treatment (P = 0.24) and depth (P = 0.14) showed no effect. Our results indicate that low-intensity laser irradiation could be a valuable non-invasive tool for the induction of wound healing in recalcitrant ulcers, and that healing time is correlated with the ulcer cause and size.
GaAs laser treatment of venous ulcers.
Soriano, F (1998)
In a study extended over 6 years Soriano treated 231 patients with venous leg ulcers. The exclusion criterias were diabetes, arterial disease, vasculitis, congestive heart failure and loss of follow up at 6 months. 122 of 154 patients in the laser group fulfilled the study. In the control group (traditional treatment only) 46 of 77 patients fulfilled. Wounds were all of Size Rate 4 or larger (diameter major + diameter minor). A 40 mW GaAs laser at 10.000 Hz was used, The laser was applied in the point technique with a dose of 3 J/cm2 per point around the border and onto the bed of the ulcer in non contact. Three sessions a week were performed for 4 months, or until the ulcer was completely healed. The results were evaluated as complete healing, partial healing (more than 50%) or non healing (less than 50%). In the laser group there was a 70% healing rate and a 14% rate of partial healing. In the control group 26% of the patients had a complete healing and 22% a partial healing. In the laser group, only 19% of the ulcers of great size (>16) healed completely and if the wound was more than one year old, the percentage of complete healing was 40%. Wounds with an oedema failed to heal with the parameters used.
Low intensity laser irradiation in the treatment of recalcitrant radiation ulcers in patients with breast cancer–long-term results of 3 cases.
Schindl, A, Schindl, M, Pernerstorfer-Schon, H, Mossbacher, U and Schindl, L (2000)
Radiotherapy can be followed by recalcitrant skin ulcers. As low intensity laser irradiation has been demonstrated to have a beneficial effect on impaired wound healing, we investigated its efficacy and safety in three patients with chronic radiation ulcers. The three patients, previously mastectomized due to breast cancer, with recalcitrant radiation ulcers of the skin were treated with a 30 mW helium-neon laser (wavelength: 632.8 nm, intensity: 3 mW/cm2, dose: 30 J/ cm2) three times weekly. In all patients, complete wound closure was achieved within a period of 7, 5, and 8 weeks. One patient died 6 weeks after laser treatment due to tumor cachexia. Neither of the other patients showed recurrence of radiation ulcers or neoplasm during a follow-up of 36 months. Low intensity helium-neon laser irradiation has been shown to be effective in the induction of wound healing in radiotherapy-induced ulcers in three patients with breast cancer.
Thermography in the Diagnosis of CRPS: A Physician’s Opinion
by Dr P Getson DO Pain Practitioner 2006 Vol. 16 No. 1 REVIEW ARTICLE FROM PARC PEARL DEC. 2006
Diagnosing CRPS is difficult at best and doctors have yet to come up with a definitive test. One helpful diagnostic tool which helps assemble the pieces of the puzzle is thermography. It has been around since the 1950’s and still is used at NASA.
Neuromuscular disorders can be diagnosed with thermography. With regard to CRPS, the infrared cameras are hi-tech computer images which measure changes in skin temperature.
“The sympathetic nervous system (SNS) controls these changes and changes in the sympathetics cause changes in the thermal imaging which do not conform to dermatomal patterns”. Thermography is exacting in measuring temperature and temperature differences.
“Thermography shows changes in skin temperature to one tenth of one degree centigrade. Lack of symmetry is out of conformation to dermatomal distributions.”
Measurements on a CRPS patient within the first six months shows the affected side to be warmer than the contralateral side by 0.9 Degrees C which is considered as standard for sympathetically maintained thermal asymmetry. Sometimes this uneven temperature is 1.5-2 degrees C. difference. After six months, the pattern changes and the affected side is the “cold side”. The temperature difference is often seen in very striking, vivid images.
While feeling the affected side with the hand measures temperature, the thermogram is much more sensitive and the temperature scale is very sensitive also. It is specifically calibrated to measure very small differences. It can be adjusted to allow for room or body temperature.
Another interesting thing to observe in “CRPS is the spreading patterns which can be seen 6-9 months prior to the occurrence of symptoms in a limb that has been affected with dysfunction but has not yet become symptomatic“. Patients mention symptoms in one limb which are seen as thermal abnormalities in other limb.
Thermography means that patients can be diagnosed and treated earlier.
USES: New cameras have real-time imaging properties that could help monitor a limb while a spinal cord stimulator is being installed. Thermography could help the surgeon place the leads accurately so that the patient gets maximum benefit from the stimulator.
DIAGNOSING CRPS: Thermography is the best tool we have to date to help us with diagnosis of CRPS. It also completely validates the symptoms described by the patient. he/she is not making it up, exaggerating or hallucinating. Earlier diagnosis means earlier treatment and a better prognosis. Thermography continues to surprise us with its uses and is a valuable help in making a diagnosis of CRPS.
Source: Pain Practitioner Vol 16 No. 1 Spring 2006 and ©PARC PEARL December 2006 p. 7.
Cellular Effects of Light Therapy
Photobiomodulation Directly Benefits Primary Neurons
Functionally Inactivated by Toxins
THE ROLE OF CYTOCHROME c OXIDASE*
Received for publication, August 23, 2004, and in revised form, November 10, 2004
Published, JBC Papers in Press, November 22, 2004, DOI 10.1074/jbc.M409650200
Margaret T. T. Wong-Riley, Huan Ling Liang, Janis T. Eells, Britton Chance,
Michele M. Henry, Ellen Buchmann, Mary Kane, and Harry T. Whelan
From the Departments of Cell Biology, Neurobiology, and Anatomy, Neurology, and Ophthalmology, Medical College
of Wisconsin, Milwaukee, Wisconsin 53226, Department of Health Sciences, University of Wisconsin, Milwaukee,
Wisconsin 53201, and Department of Biochemistry and Biophysics, University of Pennsylvania,
Philadelphia, Pennsylvania 19104-6059
ABSTRACT: Far-red and near infrared (NIR) light promotes wound healing, but the mechanism is poorly understood. Our previous studies using 670 nm light-emitting diode (LED) arrays suggest that cytochrome c oxidase, a photo acceptor in the NIR range, plays an important role in therapeutic photobiomodulation. If this is true, then an irreversible inhibitor of cytochrome c oxidase, potassium cyanide (KCN), should compete with LED and reduce its beneficial effects.
Results: This hypothesis was tested on primary cultured neurons. LED treatment partially restored enzyme activity blocked by 10–100 M KCN. It significantly reduced neuronal cell death induced by 300 M KCN from 83.6 to 43.5%. However, at 1–100 mM KCN, the protective effects of LED decreased, and neuronal deaths increased. LED significantly restored neuronal ATP content only at 10 M KCN but not at higher concentrations of KCN tested. Pretreatment with LED-enhanced efficacy of LED during exposure to 10 or 100 M KCN but did not restore enzyme activity to control levels. In contrast, LED was able to completely reverse the detrimental effect of tetrodotoxin, which only indirectly down-regulated enzyme levels. Among the wavelengths tested (670, 728, 770, 830, and 880 nm), the most effective ones (830 nm, 670 nm) paralleled the NIR absorption spectrum of oxidized cytochrome c oxidase, whereas the least effective wavelength, 728 nm, did not.
Conclusions: The results are consistent with our hypothesis that the mechanism of photobiomodulation involves the up-regulation of cytochrome c oxidase, leading to increased energy metabolism in neurons functionally inactivated by toxins.
Improvement of Pain and Disability in Elderly Patients with Degenerative Osteoarthritis of the Knee Treated with Narrow-Band Light Therapy
by Jean Stelian, M.D., Israel Gil, M.D., Beni Habot, M.D., Michael Rosenthal, M.D., Julian Abramovici, M.D., Nathalia Kutok, M.D., and Auni Khahil, M.D.
Journal of the American Geriatric Society, January 1992, Volume 40, Number 1
ABSTRACT: Objective: To evaluate the effects of low-power light therapy on pain and disability in elderly patients with degenerative osteoarthritis of the knee. Design: Partially double-blinded, full randomized trial comparing red, infrared, and placebo light emitters. Patients: 50 patients with degenerative osteoarthritis of both knees were randomly assigned to three treatment groups: red (15 patients), infrared (18 patients), and placebo (17 patients). Infrared and placebo emitters were double-blinded. Interventions: Self-applied treatment to both sides of the knee for 15 minutes twice a day for 10 days. Main Outcomes: Short-form McGill Pain Questionnaire, Present Pain Intensity, and Visual Analog Scale for pain and Disability Index Questionnaire for disability were used. We evaluated pain and disability before and on the tenth day of therapy. The period from the end of the treatment until the patient’s request to be retreated was summed up 1 year after the trial.
Results: Pain and disability before treatment did not show statistically significant differences between the three groups.Pain reduction in the red and infrared groups after the treatment was more than 50% in all scoring methods (P less than 0.05). There was no significant pain improvement in the placebo group. We observed significant functional improvement in red- and infrared-treated groups (p less than 0.05), but not in the placebo group. The period from the end of treatment until the patients required treatment was longer for red and infrared groups than for the placebo group (4.2 +/- 3.0, 6.1 +/- 3.2, and 0.53 +/- 0.62 months, for red, infrared, and placebo, respectively).
Conclusions: Low-power light therapy is effective in relieving pain and disability in degenerative osteoarthritis of the knee.
By Michael R Hamblin
J Neurosci Res. Author manuscript; available in PMC 2018 Oct 1.
Published in final edited form as:
J Neurosci Res. 2018 Apr; 96(4): 731–743.
Published online 2017 Nov 13. doi: 10.1002/jnr.24190
Abstract
There is a notable lack of therapeutic alternatives for what is fast becoming a global epidemic of traumatic brain injury (TBI). Photobiomodulation (PBM) employs red or near-infrared (NIR) light (600-1100nm) to stimulate healing, protect tissue from dying, increase mitochondrial function, improve blood flow and tissue oxygenation. PBM can also act to reduce swelling, increase antioxidants, decrease inflammation, protect against apoptosis, and modulate microglial activation state. All these mechanisms of action strongly suggest that PBM delivered to the head should be beneficial in cases of both acute and chronic TBI. Most reports have used NIR light either from lasers or from light-emitting diodes (LEDs). Many studies in small animal models of acute TBI have found positive effects on neurological function, learning and memory, and reduced inflammation and cell death, in the brain. There is evidence that PBM can help the brain to repair itself by stimulating neurogenesis, upregulating BDNF synthesis, and encouraging synaptogenesis. In healthy human volunteers (including students and healthy elderly women) PBM has been shown to increase regional cerebral blood flow, tissue oxygenation and improve memory, mood and cognitive function. Clinical studies have been conducted in patients suffering from the chronic effects of TBI. There have been reports of improvements in executive function, working memory, and improved sleep. Functional magnetic resonance imaging has shown modulation of activation in intrinsic brain networks likely to be damaged in TBI (default mode network and salience network).
1. Introduction
Photobiomodulation (PBM) formerly known as low-level laser (light) therapy (LLLT) is approaching its 50th anniversary, after being discovered by Endre Mester working in Hungary in 1967 (Hamblin et al. 2016). Originally thought to be a property of red lasers (600-700 nm), PBM has broadened to include near-infrared (NIR) wavelengths 760-1200 nm, and even blue and green wavelengths. Moreover the advent of inexpensive and safe light emitting diodes (LEDs) has supplanted the use of expensive lasers in many indications. The better tissue penetration properties of NIR light, together with its good efficacy, has made it the most popular wavelength range overall. The best-known medical applications of PBM have been for indications such as stimulation of wound healing (Hopkins et al. 2004; Kovacs et al. 1974), reduction of pain and inflammation in orthopedic and musculoskeletal conditions (Aimbire et al. 2006; Gam et al. 1993), and mitigation of cancer therapy side-effects (Zecha et al. 2016a; Zecha et al. 2016b). However in recent years there has been growing interest in the use of PBM in various brain disorders (Hamblin 2016b; Hennessy and Hamblin 2016; Naeser and Hamblin 2011; Naeser and Hamblin 2015). The almost complete lack of any adverse side-effects of PBM, coupled with growing disillusion with pharmaceutical drugs that affect brain function, have combined together to suggest an alternative physical therapy approach to improving brain function.
Traumatic brain injury (TBI) is caused by some type of trauma to the head, often resulting from road traffic accidents, assaults, falls, sports injuries, or blast injuries suffered in military conflict. TBI is classified as mild (loss of consciousness 0-30 minutes; altered mental state <24 hours; post-trauma amnesia <1 day); moderate (loss of consciousness 30 minutes to 24 hours; altered mental state >24 hours; post-trauma amnesia >1-7 days), or severe (loss of consciousness >24 hours; altered mental state >24 hours; post-trauma amnesia >7 days) (Blennow et al. 2016). There are three cases of TBI sustained each minute in the US (Faul et al. 2010). Repeated mild episodes of TBI (also known as concussions) even without loss of consciousness, may have devastating cumulative effects (Kamins and Giza 2016). Chronic traumatic encephalopathy is a recently recognized condition resulting from repeated head trauma, found in boxers, football players, and military personnel (McKee et al. 2016; Safinia et al. 2016). There is presently no accepted treatment for TBI, although some investigational approaches are being tested in both the acute (neuroprotection) and chronic (neurorehabilitation) settings (Loane and Faden 2010). One of these novel approaches is PBM or LLLT (Hamblin 2016a; Hamblin 2016b; Huang et al. 2012; Thunshelle and Hamblin 2016).
2. Mechanisms of action
Uncertainties about the mechanism of action of PBM at the molecular and cellular levels, have undoubtedly held back its acceptance in the wider biomedical community. However in recent years substantial progress has been made in this regard (de Freitas and Hamblin 2016). In the following section the state-of-the-art knowledge about the mechanisms of PBM is summarized. Figure 1 shows a graphical representation of the cellular and molecular mechanisms of PBM.
2.1 Chromophores
The first law of photobiology states that a photon must be absorbed by some molecule within the tissue to have any biological effect. The identity of these chromophores has been the subject of much scientific investigation and speculation. Largely due to the efforts of Tiina Karu in Russia, the enzyme cytochrome c oxidase (CCO) has been identified as a major chromophore of red/NIR light (Karu 1999; Karu and Kolyakov 2005; Karu et al. 2004a; Karu et al. 2004b). CCO is unit IV in the mitochondrial respiratory chain and has absorption peaks reaching well into the NIR spectral region (up to 900 nm) as well as in the red and blue regions. The most discussed hypothesis to explain exactly how photon absorption can stimulate the activity of CCO involves the photodissociation of inhibitory nitric oxide (NO) that can bind to the copper and heme centers in the enzyme and prevent oxygen from gaining access to the active sites (Lane 2006). In experimental models (such as isolated mitochondria) oxygen consumption and ATP production are increased, and the mitochondrial membrane potential is raised (Passarella et al. 1984).
A less well-appreciated mechanism involves light and heat-gated ion channels. These cation ion channels are thought to be members of the transient receptor potential (TRP) superfamily consisting of over 28 distinct members organized into six subfamilies, based on their primary amino acid structures (Caterina and Pang 2016). TRPV (vanilloid sub-family) members including TRPV1 (capsaicin receptor) have been shown to be activated by various wavelengths of light including green, red and NIR.
2.2 Cellular mechanisms
After the primary photon absorption event occurs, whether that the photons are absorbed by CCO, or by TRP ion channels a series of secondary events occurs. One of these events is the generation of reactive oxygen species (ROS), which are thought to be produced inside the mitochondria due to an increase in electron transport, and a rise in the mitochondrial membrane potential above the baseline levels (Suski et al. 2012). It should be noted that mitochondrial ROS can be produced when MMP is raised above normal, and also when ROS is reduced below normal. It is thought that the ROS produced when MMP is lowered (mitochondrial dysfunction) are more damaging than ROS produced when MMP is raised (mitochondrial stimulation). Nitric oxide is produced after PBM (Hamblin 2008), possibly by photodissociation from CCO where it inhibits oxygen consumption and electron transport (Lane 2006). Cyclic adenosine monophosphate (cAMP) (Gao and Xing 2009) and intracellular calcium are increased (Alexandratou et al. 2002). Many of these secondary mediators in the signaling pathways triggered by PBM, can induce activation of transcription factors, that go on to upregulate or downregulate expression levels of a large number of genes. One of the best-known transcription factors is NF-kB that can regulate expression of over one hundred genes including proteins with antioxidant, anti-apoptotic, pro-proliferation, and pro-migration functions. PBM (810 nm 3J/cm2) was shown to activate NF-kB in mouse embryonic fibroblasts via ROS production (Chen et al. 2011a). Since NF-kB is known to be a pro-inflammatory transcription factor, it might be thought that PBM would be pro-inflammatory. However it was shown that NF-KB was decreased in already activated (treated with Toll-like receptor ligands) inflammatory dendritic cells by PBM (810 nm 3J/cm2) (Chen et al. 2011b).
2.3 Tissue mechanisms
The changes in expression levels of proteins involved in antioxidant and redox-regulation, anti-apoptotic and pro-survival, cellular proliferation, etc mean that distinct changes in tissue homeostasis, healing and regeneration can be expected after PBM. For instance, structural proteins such as collagen are newly synthesized in order to repair tissue damage (Tatmatsu-Rocha et al. 2016). Cells at risk of dying in tissue that has been subjected to ischemic or other insults are protected (Sussai et al. 2010). Stem cells are activated to leave their niche, proliferate and differentiate (Oron and Oron 2016; Zhang et al. 2016). Pain and inflammation are reduced (Chow et al. 2009). Blood flow is increased (Samoilova et al. 2008) (possibly as a result of the release of NO (Mitchell and Mack 2013)), which also stimulates lymphatic drainage thereby reducing edema (Dirican et al. 2011).
2.4 Brain specific mechanisms
In addition to the foregoing, there are some PBM tissue mechanisms that are specific to the brain. One of the most important is an increase in cerebral blood flow often reported after transcranial photobiomodualtion (tPBM) (Salgado et al. 2015), leading to increased tissue oxygenation, and more oxidized CCO as measured by NIR spectroscopy (Rojas and Gonzalez-Lima 2013). tPBM has been shown to reduce activated microglia in the brains of TBI mice as measured by IBA1 (ionized calcium-binding adapter molecule-1) expression thus demonstrating reduced neuroinflammation (Khuman et al. 2012). tPBM has been shown to increase neurogenesis (formation of new brain cells derived from neuroprogenitor cells) (Xuan et al. 2014), and synaptogenesis (formation of new connections between existing brain cells) (Xuan et al. 2015) both in TBI mice. Figure 2 shows a graphical representation of a variety of these brain-specific tissue mechanisms.
3. Transcranial photobiomodulation
Transcranial PBM is a growing approach to many different brain disorders that may be classified as sudden onset (stroke, TBI, global ischemia), neurodegenerative (Alzheimer’s, Parkinson’s, dementia), or psychiatric (depression, anxiety, posttraumatic stress disorder)(Hamblin 2016b; Hennessy and Hamblin 2016; Thunshelle and Hamblin 2016). In the following section some issues concerning where the light should be delivered, and the effects of PBM on uninjured mice and humans are addressed.
3.1 Light penetration
Several laboratories working in the field of tissue optics, have investigated the penetration of light of different wavelengths though the scalp and the skull, and to what depths into the parenchyma of the brain this light can penetrate. Answering the question “can light shone on the head sufficiently penetrate to reach the brain?” is difficult. The main reason is that at present it is unclear exactly what threshold of power density is necessary (expressed in mW/cm2) at some depth inside the brain to have a biological effect. There clearly must be a minimum value below which, the light can be delivered for an infinite time without having any effect, but whether this threshold is in the region of μW/cm2 or mW/cm2 is unknown at present.
Haeussinger et al. estimated that the mean penetration depth (5% remaining intensity) of NIR light through the scalp and skull was 23.6 + 0:7 mm (Haeussinger et al. 2011). Other studies have found comparable results with some variations depending on the precise location on the head and the precise wavelength studied (Okada and Delpy 2003; Strangman et al. 2014).
Jagdeo et al. (Jagdeo et al. 2012) used human cadaver heads (skull with intact soft tissue) to measure penetration of 830 nm light, and found that penetration depended on the anatomical region of the skull (0.9% at the temporal region, 2.1% at the frontal region, and 11.7% at the occipital region). Tedord et al. (Tedford et al. 2015) also used human cadaver heads to compare penetration of 660 nm, 808 nm, and 940 nm light. They found that 808 nm light penetrated best, and could reach a depth in the brain of 40–50 mm. Lapchak et al. compared the transmission of 810 nm light through the skulls (no soft tissue) of four different species, and found the mouse skull transmitted 40%, while for rat it was 21%, for rabbit it was 11.3 and for the human skull it was only 4.2% (Lapchak et al. 2015). Pitzschke and colleagues compared penetration of 670 nm and 810 nm light into the brain when delivered by a transcranial or a transphenoidal approach, and found that the best combination was 810 nm delivered transphenoidally (Pitzschke et al. 2015). Yaroslavsky et al. examined light penetration of different wavelengths through different parts of the brain tissue (white brain matter, gray brain matter, cerebellum, and brainstem tissues, pons, thalamus). Best penetration was found with wavelengths between 1000 and 1100 nm (Yaroslavsky et al. 2002). Henderson and Morries found that between 0.45% and 2.90% of 810 nm or 980 nm light penetrated through 3 cm of scalp, skull and brain tissue in ex vivo lamb heads (Henderson and Morries 2015a).
3.2 Local vs systemic effects of light
It is possible that the beneficial effects of PBM on the brain cannot be entirely explained by penetration of light through the scalp and skull into the brain itself, at a sufficient intensity to have an effect on the brain cells. The surface power density that can be safely applied to the head, is limited by heating of the skin. Perceptible heating of the skin starts to be felt when the power density is over about 500 mW/cm2, and can become severe at 1 W/cm2.
There has been one study that explicitly addressed whether direct transcranial PBM or indirect PBM is best for the brain. In a study of PBM for Parkinson’s disease in a mouse model, Mitrofanis and colleagues compared the direct delivery of light to the mouse head, and they also covered up the head with aluminum foil so that the light was delivered to the remainder of the mouse body. They found that there was a highly beneficial effect on brain histology with light delivered to the head, but nevertheless there was also a statistically significant although less pronounced benefit (referred to as an “abscopal effect”) when the head was shielded from light. Moreover Oron and co-workers (Farfara et al. 2015) have shown that delivering NIR light to the mouse tibia (using either surface illumination or a fiber optic) resulted in improvements in memory and spatial learning in a transgenic mouse model of Alzheimer’s disease. They proposed the mechanism involved PBM stimulating c-kit-positive mesenchymal stem cells (MSCs) that were normally resident in autologous bone marrow. These MSCs were proposed to be able to infiltrate the brain, and clear β-amyloid plaques (Oron and Oron 2016). It should be noted in general that the calvarial bone marrow of the skull contains substantial numbers of stem cells (Iwashita et al. 2003).
3.3 PBM for brain in uninjured animals
Several laboratories have reported that shining light onto the head of uninjured healthy mice or rats can improve various cognitive and emotional parameters. The first study reported that exposure of the middle aged (12 months) CD1 female mice to 1072 nm LED arrays (Michalikova et al. 2008) produced improved performance in a 3D maze compared to sham treated age-matched controls. Gonzalez-Lima and coworkers (Gonzalez-Lima and Barrett 2014) showed that transcranial PBM (9 mW/cm2 with a 660 nm LED array) delivered to rats induced dose-dependent increases in oxygen consumption (5% after 1 J/cm2 and 16% after 5 J/cm2) [113]. They also found that tPBM reduced fear renewal and prevented the reemergence of the extinguished conditioned fear-responses (Rojas et al. 2012).
3.4 PBM for enhancement of brain function in uninjured human volunteers
Gonzalez-Lima et al delivered transcranial PBM (1064 nm laser, 60 J/cm2 at 250 mW/cm2) to the forehead in uninjured human volunteers in a placebo-controlled, randomized study. The goal was to improve performance of cognitive tasks related to the prefrontal cortex, including a psychomotor vigilance task (PVT), a delayed match-to-sample (DMS) memory task, and improved mood as measured by the positive and negative affect schedule (PANAS-X) (Barrett and Gonzalez-Lima 2013). Subsequent studies in uninjured humans showed that tPBM with 1064 nm laser could improve performance in the Wisconsin Card Sorting Task (considered the gold standard test for executive function) (Blanco et al. 2015). They also showed that tPBM to the right forehead (but not the left forehead) could improve attention bias modification (ABM) in humans with depression (Disner et al. 2016).
Salgado et al. applied transcranial LED to enhance cerebral blood flow in healthy elderly women, as measured by transcranial Doppler ultrasound (TCD) of the right and left middle cerebral artery and basilar artery. Twenty-five non-institutionalized elderly women (mean age 72 years), with cognitive status > 24, were assessed using TCD before and after transcranial LED therapy. tPBM (627 nm, 70 mW/cm2, 10 J/cm2) was performed at four points of the frontal and parietal region for 30 s each twice a week for 4 weeks. There was a significant increase in the systolic and diastolic velocity of the left middle cerebral artery (25 and 30%, respectively) and the basilar artery (up to 17 and 25%), as well as a decrease in the pulsatility index and resistance index values of the three cerebral arteries analyzed (Salgado et al. 2015).
3.5 PBM for acute stroke
Transcranial PBM delivered to the head, has been investigated as a possible treatment for acute stroke (Lapchak 2010). Animal models such as rats and rabbits, were first used as laboratory models, and these animals had experimental strokes induced by a variety of methods and were then treated with light (usually 810 nm laser) within 24 h of stroke onset (Lampl 2007). In these studies intervention by tLLLT within 24 h had meaningful beneficial effects.
Treatment of acute stroke in human patients was then addressed in a series of three clinical trials called “Neurothera Effectiveness and Safety Trials” (NEST-1 (Lampl et al. 2007), NEST-2 (Huisa et al. 2013), and NEST-3 (Zivin et al. 2014)). The protocol used an 810 nm laser applied to the shaved head (20 separate points in the 10/20 EEG system) within 24 h of patients suffering an ischemic stroke. The first study, NEST-1, enrolled 120 patients between the ages of 40 to 85 years of age and found a significantly improved outcome (p < 0.05 real vs sham, NIH Stroke Severity Scale) 5 days after a single laser treatment had been administered (Lampl et al. 2007). This significantly improved status was still present 90 days post-stroke in 70% of the PBM patients (but only 51% of the sham patients). The second clinical trial, NEST-2, enrolled 660 patients, aged 40 to 90, who were randomly assigned to one of two groups (331 to PBM, 327 to sham) (Zivin et al. 2009). Significant improvements (p < 0.04) were found in the moderate and moderate-severe (but not for the severe) stroke patients. The last clinical trial, NEST-3, was planned for 1000 patients enrolled, but the study was prematurely terminated by the DSMB for futility (an expected lack of statistical significance) (Lapchak and Boitano 2016). Many commentators have asked how tPBM could work so well in the first trial, yet fail in the third trial. Insufficient light penetration, too long an interval between stroke onset and PBM, inappropriate stroke severity measurement scale, use of only one single tPBM treatment, and failure to illuminate different specific areas of the brain for individual patients, have all been suggested as contributory reasons (Hamblin 2016b). It is undoubtedly the case that the failure of NEST-3 has cast a cloud over the whole application of PBMT for TBI as well as for stroke. Many commentators have asked “Why are you testing PBMT for TBI, if it has been shown not to work for stroke?” The failure of the investigators not to take into account the anatomical location of the stroke (and also whether it was deep or superficial) was also likely to have played a role in the failure of NEST-3. It is logical that light should be applied to the same side of the head where the lesion was located, not both sides of the head (Naeser et al. 2012). In my opinion the use of a single application of PBMT also bore some of the responsibility. Although a single application of PBM to the head works very well for experimental animals (mice, rats, rabbits) who have suffered a stroke or a TBI, the same may not apply to humans.
4. Animal studies of PBM in acute TBI models
4.1 Studies from the Oron laboratory
Oron’s group was the first (Oron et al. 2007) to demonstrate that a single exposure of the head of a mouse a few hours after creation of a TBI lesion using a NIR laser (808 nm) could improve neurological performance and reduce the size of the brain lesion. A weight-drop device was used to induce a closed-head TBI in the mice. An 808 nm diode laser with two energy densities calculated at the surface of the brain (1.2-2.4 J/cm2 delivered by 2 minutes of irradiation with 200mW laser power to the scalp) was delivered to the head 4 hours after TBI was induced. Neurobehavioral function was assessed by the neurological severity score (NSS). There was no significant difference between the control and laser-treated group in NSS between the power densities (10 vs 20 mW/cm2), and no significant difference at early time points (24 and 48 hours) post TBI. However, there was a significant improvement (27% lower NSS score) in the PBM group at times between 5 days and 4 weeks. The laser treated group also showed a smaller loss of cortical tissue than the sham group (Oron et al.). In another study (Oron et al. 2012) they varied the pulse parameters (CW, 100Hz, or 600Hz) and tested whether the tPBM was equally effective when delivered at 4, 6, or 8 hours post-TBI. They first established that a calculated dose to the cortical surface of 1.2 J/cm2 of 808nm laser at 200mW applied to the head, was more effective when delivered at 6 hours post TBI than at 8 hours. They then selected an even shorter time post-TBI (4 hours) and compared CW with 100Hz and 600Hz. At 56 days, more mice in the 100Hz group (compared to the CW and 600 Hz groups) had fully recovered. The 600Hz group had lower NSS scores than the CW and 100Hz groups up to 20 days. Magnetic resonance imaging (MRI) analysis demonstrated significantly smaller lesion volumes in PBM-treated mice compared to controls.
4.2 Studies from the Hamblin laboratory
Wu et al. (Wu et al. 2012) first explored the effect of varying the laser wavelengths of PBM had on closed-head TBI in mice. Mice were randomly assigned to a PBM treatment group with a particular wavelength, or to a sham treatment group as a control. Closed-head injury (CHI) was induced via a weight- drop apparatus. To analyze the severity of the TBI, the neurological severity score (NSS) was measured and recorded. The injured mice were then treated with varying wavelengths of laser light (665, 730, 810 or 980 nm) at an energy density of 36 J/cm2 directed onto the scalp at 4 hours post-TBI. The 665 nm and 810 nm laser groups showed significant improvement in NSS when compared to the control group between days 5 to 28. By contrast, the 730 nm and 980 nm laser groups did not show any significant improvement in NSS (Wu et al. 2012) (Figure 3). The tissue chromophore cytochrome c oxidase (CCO) is proposed to be responsible for the underlying photon absorption process that underlies many PBM effects. CCO has absorption bands around 665 nm and 810 nm while it has a low absorption region at the wavelength of 730 nm (Karu et al.). It should be noted that this particular study (Wu et al. 2012) found that the 980 nm did not produce the same positive effects as the 665 nm and 810 nm wavelengths did; nevertheless previous studies did find that the 980 nm wavelength was an active one for PBM (Anders et al. 2014). Wu et al. suggested that these dissimilar results may be due to differences in the energy density, irradiance etc. between the other studies and the Wu study (Wu et al. 2012). In particular a much lower dose of 980 nm might have been effective had it been tested (Wang et al. 2016). Ando et al. (Ando et al. 2011) next used the 810 nm wavelength produced by a Ga-Al-As diode laser delivered at parameters used in the Wu study, and varied the pulse modes of the laser. These modes consisted of either pulsed wave at 10 Hz or at 100 Hz (50% duty cycle) or continuous wave laser. They used a different mouse model of TBI induced with a controlled cortical impact device directly inflicting a lesion on the cortex via an open craniotomy. A single treatment with a power density of 50 mW/m2 and an energy density of 36 J/cm2 (duration of 12 minutes) was given via tLLLT to the closed head in mice at 4 hours post CCI. At 48 hours to 28 days post TBI, all laser treated groups had significant decreases in the measured neurological severity score (NSS) when compared to the controls. Although all laser treated groups had similar NSS improvement rates up to day 7, the PW 10 Hz group began to show even greater improvement beyond this point as seen in Figure 4. At day 28, the forced swim test for depression and anxiety was used and showed a significant decrease in the immobility time for the PW 10 Hz group. In the tail suspension test, which measures depression and anxiety, there was also a significant decrease in the immobility time at day 28, and also at day 1, in the PW 10 Hz group.
Studies using immunofluorescence staining of sections cut from mouse brains showed that tPBM increased neuroprogenitor cells (incorporating BrdU) in the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) at 7 days after the treatment (Xuan et al. 2014). The neurotrophin known as brain derived neurotrophic factor (BDNF) was also increased in the DG and SVZ at 7 days, while the protein marker (synapsin-1) for synaptogenesis and neuroplasticity was increased in the cortex at 28 days but not in the DG, SVZ or in any location at 7 days (Xuan et al. 2015). Learning and memory as measured by the Morris water maze was also improved by tPBM (Xuan et al. 2014).
4.3 Studies from the Wu laboratory
Zhang et al. (Zhang et al. 2014) first showed that secondary brain injury occurred to a worse degree in mice that had been genetically engineered to lack “Immediate Early Response” gene X-1 (IEX-1). When these mice were exposed to a gentle head impact (thought to closely resemble mild TBI in humans) they had a worse NSS than uninjured mice with the same TBI. Exposure of IEX-1 knockout mice to PBM (150 mW/cm2, 4 min, and 36 J/cm2) delivered at 4 hours post injury, restored the NSS to almost baseline levels, suppressed proinflammatory cytokine expression of interleukin (IL)-Iβ and IL-6, but upregulated TNF-α. The original lack of IEX-1 decreased ATP production, but exposing the injured brain to LLLT elevated ATP production back to near normal levels.
Dong et al. (Dong et al. 2015) asked whether the beneficial effects of PBM on TBI in mice could be enhanced by combining PBM with administration of metabolic substrates such as pyruvate and/or lactate. The goal was to even further improve mitochondrial function in the brain. This combinatorial treatment was able to reverse memory and learning deficits in TBI injured mice back to normal levels as well as leaving the hippocampal region completely protected from tissue loss; a stark contrast to control TBI mice that exhibited severe tissue loss from secondary brain injury.
4.4 Studies from the Whalen laboratory
Khuman et al (Khuman et al. 2012) delivered PBM (800nm) either directly to the injured brain tissue (through the craniotomy) or transcranially in mice beginning 60-80 min after CCI TBI. At a dose of 60J/cm2 (500mW/cm2) the mice showed increased performance in the Morris water maze (latency to the hidden platform, p<0.05, and probe trial, p<0.01) compared to non-treated controls. When PBM was delivered via open craniotomy there was reduced microgliosis at 48h (IbA-1+ cells, p<0.05). Little or no effect of tPBM on post-injury cognitive function was observed using lower or higher doses, a 4-h administration time point or 60J/cm2 at 7-days post-TBI.
4.5 Studies from the Whelan laboratory
Quirk et al (Quirk et al. 2012) studied Sprague-Dawley rats who had received a severe CCI TBI and were divided into three groups: real TBI, sham surgery, and anesthetization only. Each group received either real or sham PBM consisting of 670nm LED treatments of 15J/cm2, 50mW/cm2, 5min, given two times per day for 3 days (chemical analysis) or 10 days (behavioral analysis using a TruScan nose-poke device). Significant differences in task entries, repeat entries, and task errors were seen in the TBI rats treated with PBM vs untreated TBI mice, and in sham surgery mice treated with PBM vs untreated sham surgery mice. A statistically significant decrease was found in the pro-apoptotic marker Bax, and increases in the anti-apoptotic marker Bcl-2 and reduced glutathione (GSH) levels in tPBM TBI mice.
4.6 Studies from the Marques laboratory
Moreira et al used a different model of TBI (Moreira et al. 2009). Wistar rats received a craniotomy and a copper probe cooled in liquid nitrogen was applied to the surface of the brain to create a standardized cryogenic injury. They treated the rats with either a 780nm or 660nm laser at one of two different doses (3J/cm2 or 5J/cm2) twice (once immediately after the injury and again 3 hours later). Rats were sacrificed 6h and 24h after the injury. The 780nm laser was better at reducing levels of pro-inflammatory cytokines (TNFα, IL1β, IL6) particularly at early timepoints (Moreira et al. 2009). In a follow-up study using 3 J/cm2 (Moreira et al. 2011) these workers reported on the healing of the injuries in these rats at timepoints 6h, 1, 7 and 14 days after the last irradiation. Cryogenic injury created focal lesions in the cortex characterized by necrosis, edema, hemorrhage and inflammatory infiltrate. The most striking findings were: PBM-treated lesions showed less tissue loss than control lesions at 6h. During the first 24h the amount of viable neurons was significantly higher in the PBM groups. PBM reduced the amount of GFAP (glial fibrillary acidic protein, a marker of astrogliosis) and the numbers of leukocytes and lymphocytes, thus demonstrating its anti-inflammatory effect.
5. Patients with chronic TBI
The majority of studies of PBM for TBI in laboratory animals have been conducted in the acute setting, while the majority of human studies of PBM for TBI have been conducted in patients who have suffered head injuries at various times in the past (sometimes quite a long time ago).
5.1 Naeser case reports
In 2011 Naeser, Saltmarche et al., published the first report describing two chronic, TBI cases treated with tPBM (Naeser et al. 2011). A 500 mW CW LED source (mixture of 660 nm red and 870 nm NIR LEDs) with a power density of 22.2 mW/cm2 (area of 22.48 cm2), was applied all over the head, for 10 minutes at each placement location (13.3 J/cm2). In the first case study the patient reported that she could concentrate on tasks for a longer period of time (the time able to work at a computer increased from 20 minutes to 3 hours). She had a better ability to remember what she read, decreased sensitivity when receiving haircuts in the spots where PBM was applied, and improved mathematical skills after undergoing PBM. The second patient had statistically significant improvements compared to prior neuropsychological tests after 9 months of treatment. The patient had a 2 standard deviation (SD) increase on tests of inhibition and inhibition accuracy (9th percentile to 63rd percentile on the Stroop test for executive function and a 1 SD increase on the Wechsler Memory scale test for the logical memory test (83rd percentile to 99th percentile) (Naeser et al. 2011).
5.2 Naeser case series
Naeser et al then went on to report a case series containing a further eleven patients (Naeser et al. 2014). This was an open protocol study that examined whether scalp application of red and NIR LED could improve cognition in patients with chronic, mild TBI (mTBI). This study enrolled 11 participants ranging in age from 26 to 62 years (6 males, 5 females) who suffered from persistent cognitive dysfunction after mTBI. The injuries in the participants had been caused by motor vehicle accidents, sports related events and for one participant, an improvised explosive device (IED) blast. tPBM consisted of 18 sessions (Monday, Wednesday, and Friday for 6 weeks) and was started anywhere from 10 months to 8 years post-TBI. A total of 11 LED cluster heads (5.25 cm in diameter, 500 mW, 22.2 mW/cm2, 13 J/cm2) were applied for 10 minutes per set (5 or 6 LED placements per set, Set A and then Set B, in each session). Neuropsychological testing was performed pre-LED application and 1 week, 1 month and 2 months after the final treatment. They found that there was a significant positive linear trend for the Stroop Test for executive function, in trial 3 inhibition (p = 0.004); Stroop, trial 4 inhibition switching (p = 0.003); California Verbal Learning Test (CVLT)-II, total trials 1-5 (p = 0.003); CVLT-II, long delay free recall (p = 0.006). Improved sleep and fewer post-traumatic stress disorder (PTSD) symptoms, if present beforehand, were observed after treatment. Participants and family members also reported better social function and a better ability to perform interpersonal and occupational activities. Although these results were significant, the authors suggested that further placebo-controlled studies would be needed to ensure the reliability of this approach (Naeser et al. 2014).
Naeser has proposed (Naeser et al. 2016; Naeser et al. 2014) that specific scalp placements of the LED cluster heads may affect specific cortical nodes in the intrinsic networks of the brain, such as the default mode network (DMN), the salience network (SN), and the central executive network (CEN). These intrinsic networks are often dysregulated after TBI (Sharp et al. 2014). Naeser proposed that the specific areas of the head to receive light, to target cortical nodes in these networks were as follows:
For the DMN, placement of the LED cluster head on the midline of face, centered on the upper forehead and the front hairline, targeted the left and right mesial prefrontal cortex; and on a midline, scalp location half-way between the occipital protuberance and the vertex of the head, targeted the precuneus; and on left and right LED placements superior to the tip of each ear and posterior to each ear, targeted the inferior parietal cortex/angular gyrus areas.
For the SN, placement of LED cluster heads on the left and right temple areas, to target the anterior insula (but due to depth of insula, unknown if the photons reached the target); midline of the vertex of the head, to target the left and right presupplementary motor areas; and the LED cluster head placed on the midline of face, centered on the upper forehead and the front hairline, also targeted the left and right dorsal anterior cingulate cortex.
For the CEN, left and right scalp LED placements immediately posterior to the front hairline (on a line directly superior from the pupils of the eyes), targeted the dorso-lateral prefrontal cortex areas; and the left and right LED placements superior to the tip of each ear and posterior to each ear, also targeted the posterolateral inferior parietal cortex/angular gyrus areas (also treated as part of the DMN).
Further studies from Naeser and colleagues (Naeser et al. 2016) tested an intranasal LED (iLED) device. Two small iLEDs (one red and the other NIR) were clipped into each nostril and used at the same time for 25 min. The parameters were as follows: red, 633nm, 8mW CW, 1 cm2, energy density 12 J/cm2 (25 min); NIR 810nm, 14.2mW, pulsed 10Hz, 1cm2, 21.3J/cm2. The first mTBI participant (24-year old female) who had sustained four sports-related concussions (two during snowboarding and two during field hockey), received iLED PBM three times per week for 6 weeks. Significant improvements were observed in tasks measuring executive function and verbal memory as well as attention and verbal fluency. At 1 week after the 18th iLED treatment, the average total time asleep had increased by 61 min per night and her sleep efficiency (total sleep time divided by total time in bed) had increased by 11%. At 12 weeks after the last iLED treatment, she was able to discontinue all sleep medications that she had previously been using. The second, mTBI participant who received the intranasal only, LED treatment series is a 49 Yr. M (non-Veteran) who sustained mTBI in a MVA, 30 years prior to receiving the intranasal LED treatment series. He showed significant improvement on the Controlled Oral Word Association-FAS Test post- the iLED treatment series, improving by +1.3 SD and +1.5 SD at 1 and 2 months post- the 18th iLED treatment. His sleep data indicated he was already a good sleeper, at entry.
5.3 Bogdanova and Naeser studies
Bogdanova reported (Bogdanova et al. 2014) a case report of two patients (1 female) with moderate TBI (medical records and clinical evaluation) and persistent cognitive dysfunction (as measured by neuropsychological tests of executive function and memory). Patients received 18 sessions of transcranial LED therapy (3×/week for 6 weeks) using the mixed red/NIR cluster described above (Naeser et al. 2011).
Standardized neuropsychological tests for executive function, memory, depression, PTSD and sleep measures (PSQI, actigraphy) were administered to participants pre-(T1), mid-(T2), and one week (T3) post-PBM treatment. Both PBM treated cases (P1 and P2) showed marked improvement in sleep (actigraphy total sleep) 1 week post-LED treatment (T3), as compared to pre-treatment (T1). P1 also improved in executive function, verbal memory, and sleep efficiency; while P2 significantly improved on measures of PTSD (PCL-M) and depression. No adverse events were reported.
5.4 Studies from Henderson and Morries
Henderson and Morries (Henderson and Morries 2015b) used a high-power NIR laser (10-15 W at 810 and 980 nm) and applied it to the head to treat a patient with moderate TBI. The patient received 20 NIR applications over a 2-month period. They carried out anatomical magnetic resonance imaging (MRI) and perfusion single-photon emission computed tomography (SPECT). The patient showed decreased depression, anxiety, headache, and insomnia, whereas cognition and quality of life improved, accompanied by changes in the SPECT imaging.
They next reported (Morries et al. 2015) a series of ten patients with chronic TBI (average time since injury 9.3 years) where each patient received ten treatments over the course of 2 months using a high-power NIR laser (13.2 W/0.89 cm2 equivalent to 14.6 W/cm2 at 810nm; or 9 W/0.89 cm2 equivalent to 10.11 W/cm2 at 980nm). A continuous sweeping motion over the forehead was utilized to minimize skin heating and cover a larger area. Skin temperature increased no more than 3°C. Overall symptoms of headache, sleep disturbance, cognition, mood dysregulation, anxiety, and irritability improved. Symptoms were monitored by depression scales and a novel patient diary system specifically designed for this study. These authors have proposed that high power lasers are preferable for tPBM treatments because the photons can better reach the brain (Henderson and Morries 2015a).
5.5 Case study from Nawashiro
Nawashiro et al (Nawashiro et al. 2012) treated a single patient who had suffered a severe TBI. The patient survived but was left in a persistent vegetative state for 8 months after the accident. He showed no spontaneous movement of limbs and a CT scan of the head 8 months after the accident showed a focal low-density area in the right frontal lobe. The device had 23 individual 850nm LEDs (13mW each; total power 299mW, total area 57cm2). A treatment time of 30 min per session delivered 20.5 J/cm2 over the left and right forehead areas repeated twice daily (6h apart), for 73 days. Five days after beginning the PBM (after 10 treatments), the patient began to spontaneously move his left arm and hand, which had not occurred during the previous 8 months. Single-photon emission computed tomography with N-isopropyl-[123I]p-iodoamphetamine (IMP-SPECT) was performed twice. The IMP-SPECT scans showed a focal increase (20% higher) in cerebral blood flow in the uninjured left anterior frontal lobe 30 min after the last (146th) PBM treatment, compared to before PBM began.
6. Conclusion and future prospects
As was mentioned above, one of the most important questions to be answered when contemplating clinical treatment of TBI patients with tPBM, is what is the best time to administer the treatment? All the available reports of studies using PBM in laboratory animal models of TBI and stroke, and also in patients treated for stroke, have been in the acute phase where the overall goal of the intervention can be best described as neuroprotection. Not only that but there are several reports (Lapchak et al. 2007; Oron et al. 2012) that PBM for both TBI and stroke is most effective when it is delivered as soon as possible after the actual event (head impact or ischemic stroke). The protocols for the series of NEST clinical trials specified that patients should be treated with PBM within 24 hours of the stroke occurring. By contrast, all the clinical trials of PBM for patients with TBI, that have so far been carried out, have been with chronic TBI, after varying periods of time having elapsed after the original head injury, sometimes as long as 8 years. Although it would be generally supposed that tPBM would be effective when delivered to acute TBI patients, this has not yet been actually tested. If tPBM were to be used for acute TBI patients, then presumably the PBM should be delivered perhaps beginning at 4 to 6 hours post-TBI, for a limited number of times after the injury; perhaps once a day for 7 days?
The dosimetry and optimum delivery apparatus of tPBM is still uncertain. Although there is some consensus that wavelengths in the region of 800-900nm will penetrate the scalp and skull, other workers have used longer NIR wavelengths, 980nm, 1064nm, or 1072nm. Pulsing or CW is another unresolved question. The exact locations on the head that should receive the light are still unknown. Naeser has proposed (Naeser et al. 2016) some interesting considerations regarding the scalp placements of the tLEDS, and their effect on various intrinsic cortical networks of the brain. Targeted LED placements could promote better neuromodulation (activation/deactivation) in specific cortical nodes. It is possible that communication between nodes within one single network, and/or across networks could be improved. Moreover preliminary data indicate that intranasal, red plus near-infrared LEDs can also benefit TBI patients, although the degree to which light incident on the nasal mucosa, and possibly delivered transsphenoidally (Pitzschke et al. 2015) can penetrate directly into the brain, remains to be determined.
An advantage of intranasal and/or transcranial LED PBM therapy is that it can be performed in the home, for long-term use (Naeser et al. 2011). Also, 5 chronic, mild to moderately-severe dementia cases recently showed significant improvement on the Mini-Mental State Examination (p<0.003), and on the Alzheimer’s Disease Assessment Scale-Cognitive subscale (p<0.023) after 12 weeks of daily, at-home, intranasal, near-infrared LED PBM treatments (810nm, pulsed at 10 Hz), and once-a-week in-office, tLED treatments applied to the cortical nodes of the Default Mode Network (Saltmarche et al. 2017). Anecdotally, there was also improved sleep, fewer angry outbursts, and less wandering. When all LED treatments were withdrawn after 12 weeks of active LED PBM treatment, there was precipitous decline in cognition and behavior. Thus, at-home, long-term use of iLED plus tLED PBM offers a potential therapy to mitigate the sequelae of Alzheimer’s disease and possibly other neurodegenerative disorders, as well as TBI and stroke.
One highly distressing aspect of TBI symptomatology that has not so far been addressed by PBM, is that of post-traumatic epilepsy (PTE). TBI is the most significant cause of symptomatic epilepsy in people from 15 to 24 years of age. The frontal and temporal lobes are the most frequently affected regions, but imaging (MRI) often fails to show the precise cause. During PTE seizures there is an abnormal electrical discharge in the brain, with staring and unresponsiveness, stiffening or shaking of the body, legs, arms or head; strange sounds, tastes, visual images, feelings or smells; inability to speak or understand, etc (Cotter et al. 2017). Epilepsy has traditionally been considered to be a contra-indication for PBMT (Navratil and Kymplova 2002). However the knowledge that has recently been gained concerning the beneficial effects of PBMT on the damaged brain, suggests that this view may need to be critically revisited.
Moreover there is also potential of tPBM to treat a wide range of brain disorders only loosely associated with TBI, including Parkinson’s disease (Purushothuman et al. 2013), depression, anxiety, post-traumatic stress disorder, autism spectrum disorder and so on (Hamblin 2016b).
The ongoing and accelerating clinical research efforts in testing PBM for TBI, are expected to lead to the answering of many of these questions in the coming years.
For the full article please click here